Podolak, Levi, Vazan, Malamud 2023
https://ui.adsabs.harvard.edu/abs/2023Icar..39415424P/abstract
Although carbon monoxide (CO) is an abundant molecule and may have great importance for planetary interiors, measurements of its properties are difficult due to its extreme volatility. We calculate the equation of state for CO over a range of temperature and density that is applicable to the conditions in planetary interiors. Previous experimental and theoretical studies cover only a limited temperature-density range. Our calculations match these early results well, but now cover the full range of relevance. The method of calculation is based on the general-purpose quotidian equation of state described by More et al. (1988), which is here used in order to generate a freely downloadable look-up table to be used by the community.
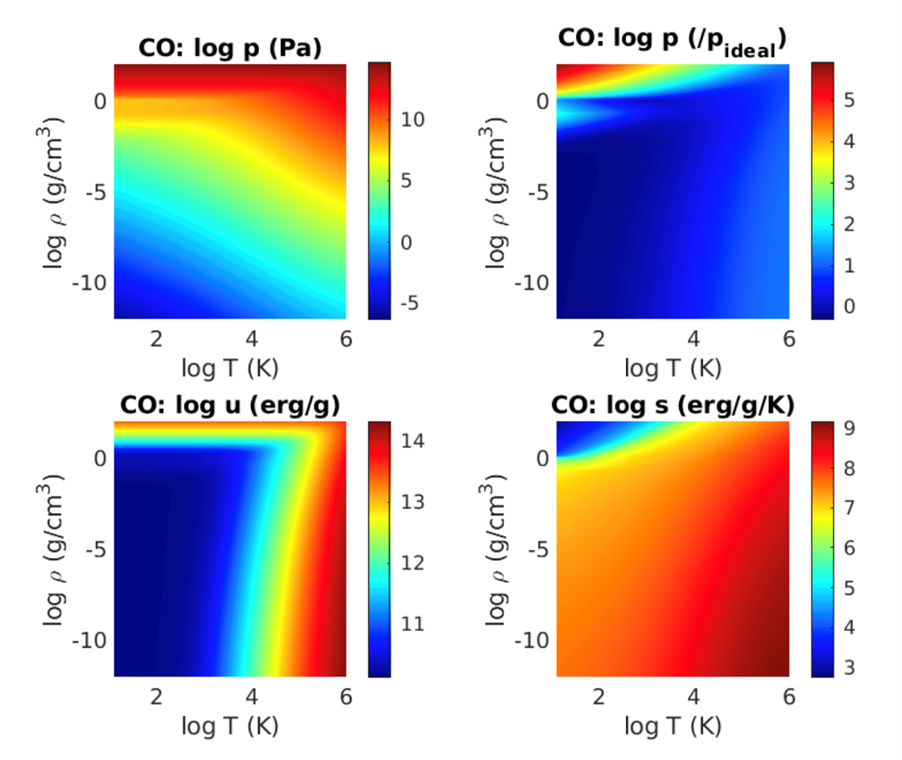
Thermodynamic properties of CO as a function of density and temperature as computed from the quotidian equation of state. Upper left: total pressure. Upper right: pressure divided by ideal gas pressure. This shows the region where an ideal gas approximation may be used. Lower left: specific internal energy. Lower right: specific entropy.